















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
A Comprehensive Guide To Simple Compound Nomenclature: Unraveling The Secrets of Chemical Naming

If you have ever dabbled in chemistry or encountered chemical compounds in any way, you may have noticed how their names seem like a complex jumble of letters and numbers. Fear not, for this comprehensive guide will walk you through the intricacies of simple compound nomenclature.
Understanding the Basics
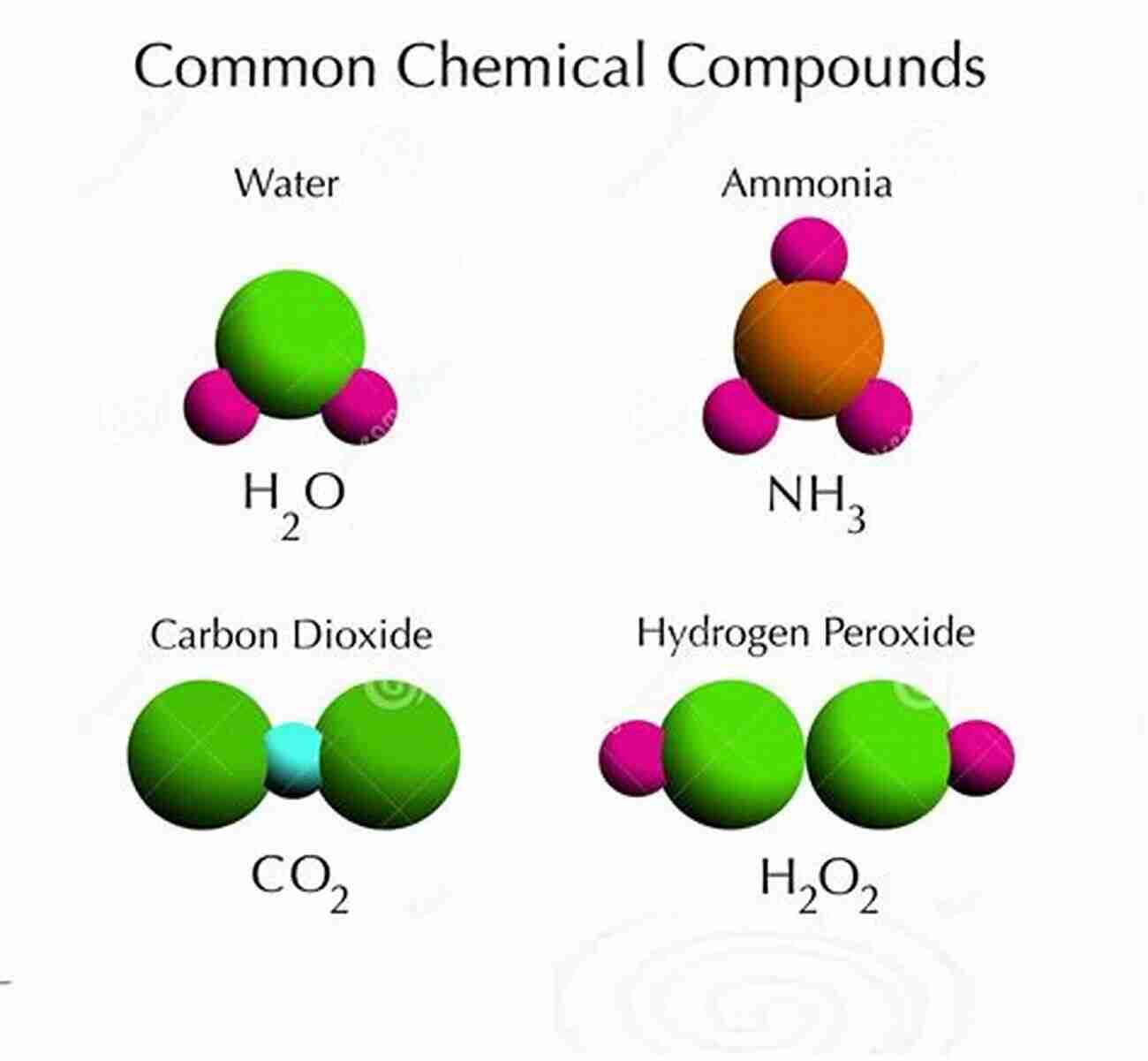
Before we dive into the world of compound naming, it is essential to understand a few key concepts. Chemical compounds consist of different elements, which are represented by symbols such as H for hydrogen, O for oxygen, and C for carbon. These elements combine in specific ratios to form compounds.
The Role of Ions and Atoms
Ions play a vital role in compound nomenclature. They are electrically charged atoms that have gained or lost electrons. Positively charged ions are called cations, while negatively charged ions are called anions.
5 out of 5
| Language | : | English |
| File size | : | 7003 KB |
| Screen Reader | : | Supported |
| Print length | : | 19 pages |
| Hardcover | : | 360 pages |
| Item Weight | : | 9 ounces |
| Dimensions | : | 8.5 x 0.18 x 11 inches |
| Paperback | : | 75 pages |
Atoms, on the other hand, are the building blocks of matter. They consist of a nucleus, which houses protons and neutrons, and electrons that orbit around the nucleus.
The Naming Process
Now that we have laid the foundation, let's delve into the process of naming simple compounds. These compounds can be broadly classified into two types: binary compounds and compounds containing polyatomic ions.
Binary Compounds
Binary compounds are composed of only two elements. The element with the positive charge always comes first, followed by the element with the negative charge. The names of the elements may be modified based on certain rules.
Rule #1: Naming Ionic Binary Compounds
When naming ionic binary compounds, the cation is always named first using its elemental name. The anion, on the other hand, is named using the first syllable of its elemental name followed by the suffix "-ide".
For example, consider the compound containing sodium (Na) and chlorine (Cl). Sodium, being the cation, is named as it is. However, chlorine, as the anion, becomes chloride when combined with sodium, resulting in the name "sodium chloride".
Rule #2: Naming Covalent Binary Compounds
Covalent binary compounds consist of two nonmetals. In these cases, prefixes are used to denote the number of atoms present for each element.
For example, the compound formed by carbon (C) and oxygen (O) is typically known as carbon monoxide (CO),where "mono" signifies one atom of oxygen.
Compounds Containing Polyatomic Ions
Polyatomic ions are charged chemical species composed of several atoms bonded together. Unlike binary compounds, the names of compounds containing polyatomic ions require some additional attention.
Rule #3: Naming Compounds with a Polyatomic Ion
When a compound contains a polyatomic ion, the name of the cation remains the same as before, while the name of the anion may change slightly.
For instance, consider the compound formed by combining sodium (Na) and nitrate (NO3-) ions. The cation remains unchanged, but the anion replaces the "-ate" suffix with "-ite", resulting in the name "sodium nitrite".
Rule #4: Naming Acids
Acids are compounds that dissociate in water to produce hydrogen (H+) ions. The names of acids depend on the anion present.
If the anion ends with "-ide", the acid is named with the prefix "hydro-" followed by the anion name with the suffix "-ic acid". For example, hydrochloric acid (HCl) is formed when hydrogen combines with chloride.
If the anion ends with "-ate" or "-ite", the acid name is derived from the root of the anion name where "-ate" becomes "-ic acid" and "-ite" becomes "-ous acid". For instance, sulfuric acid (H2SO4) corresponds to the sulfate anion (SO4^2-),while sulfurous acid (H2SO3) is derived from the sulfite anion (SO3^2-).
Congratulations! You have now successfully navigated the complex realm of simple compound nomenclature. Understanding the rules and patterns discussed in this comprehensive guide will empower you to decipher chemical compound names with ease.
Remember that practice makes perfect, so don't hesitate to dive into some chemical naming exercises to solidify your knowledge. The world of chemistry awaits you, and armed with this newfound knowledge, you'll be able to navigate it with confidence!
5 out of 5
| Language | : | English |
| File size | : | 7003 KB |
| Screen Reader | : | Supported |
| Print length | : | 19 pages |
| Hardcover | : | 360 pages |
| Item Weight | : | 9 ounces |
| Dimensions | : | 8.5 x 0.18 x 11 inches |
| Paperback | : | 75 pages |
"Bonding in Floweclature: A Guide to Simple Compound Nomenclature" is a book that explains the rules of naming simple chemical compounds in the form of a children's book! An idea sprouting from a high school chemistry project, the book explains these rules through an analogy of flowers. If you are struggling with simple compound nomenclature, I would recommend this book for you. The book explains the nomenclature rules using simple language and also provides illustrations and examples to aid the reader. In addition, reference tools such as a periodic table, a polyatomic ions list, and a nomenclature rules flowchart are included to further help the reader. I hope you enjoy reading this book!

 Calvin Fisher
Calvin FisherThe Most Insightful and Liberating Experiences Found in...
When it comes to expanding our...

 D'Angelo Carter
D'Angelo CarterDax To The Max Imagination: Unlock the Power of...
Welcome to the world of Dax To...

 Chris Coleman
Chris ColemanThe Hidden Case of Ewan Forbes: Uncovering the Mystery...
Ewan Forbes: a...

 Morris Carter
Morris CarterWhen Newport Beat New Zealand: A Historic Rugby Upset
The rivalry between Newport and New Zealand...

 David Mitchell
David MitchellThe Soul of an Astronomer: Women of Spirit
Astronomy, the study of...

 Ethan Gray
Ethan GrayThe Military Origins Of The Republic 1763-1789
When we think about the birth of the...

 Guy Powell
Guy PowellRPO System for 10 and 11 Personnel: Durell Fain
When it comes to...

 Evan Hayes
Evan HayesMadness: The Ten Most Memorable NCAA Basketball Finals
College basketball fans eagerly await the...

 Jorge Amado
Jorge AmadoDiscover the Magic of Polish: English First 100 Words,...
Are you ready to embark on a linguistic...

 Shaun Nelson
Shaun NelsonUnlock the Secrets of Edwidge Danticat's Breath, Eyes,...
Are you delving into the world...

 Walt Whitman
Walt Whitman300 Years Liechtenstein: The Birth of Fish Out of Water...
Once upon a time, in the...

 Jaden Cox
Jaden CoxExploring the Legendary Surfers of Early Surfing in the...
Surfing, a sport...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 David MitchellTyranny And Heartbreak The Complete Trilogy: An Epic Journey of Hope and...
David MitchellTyranny And Heartbreak The Complete Trilogy: An Epic Journey of Hope and... Ted SimmonsFollow ·12.9k
Ted SimmonsFollow ·12.9k Cason CoxFollow ·11k
Cason CoxFollow ·11k Andres CarterFollow ·14.1k
Andres CarterFollow ·14.1k Henry GreenFollow ·12k
Henry GreenFollow ·12k Rodney ParkerFollow ·5.3k
Rodney ParkerFollow ·5.3k Harold PowellFollow ·7.7k
Harold PowellFollow ·7.7k Isaiah PriceFollow ·19.7k
Isaiah PriceFollow ·19.7k José MartíFollow ·6.5k
José MartíFollow ·6.5k