


















Do you want to contribute by writing guest posts on this blog?
Please contact us and send us a resume of previous articles that you have written.
The Physical Basis Of Biochemistry: Unveiling the Secrets of Life


Biochemistry, often referred to as the "chemistry of life," is the field of science that explores the chemical processes within and related to living organisms. It offers a fascinating glimpse into the delicate balance of molecules, energy, and life itself. In this article, we will delve into the physical basis of biochemistry, uncovering the fundamental principles that govern the intricate mechanisms at play in every living cell.
The Building Blocks of Life
At the heart of biochemistry lie the molecules that form the basis of life. These include carbohydrates, lipids, proteins, and nucleic acids. Each of these molecules plays a crucial role in the structure, function, and regulation of living organisms.
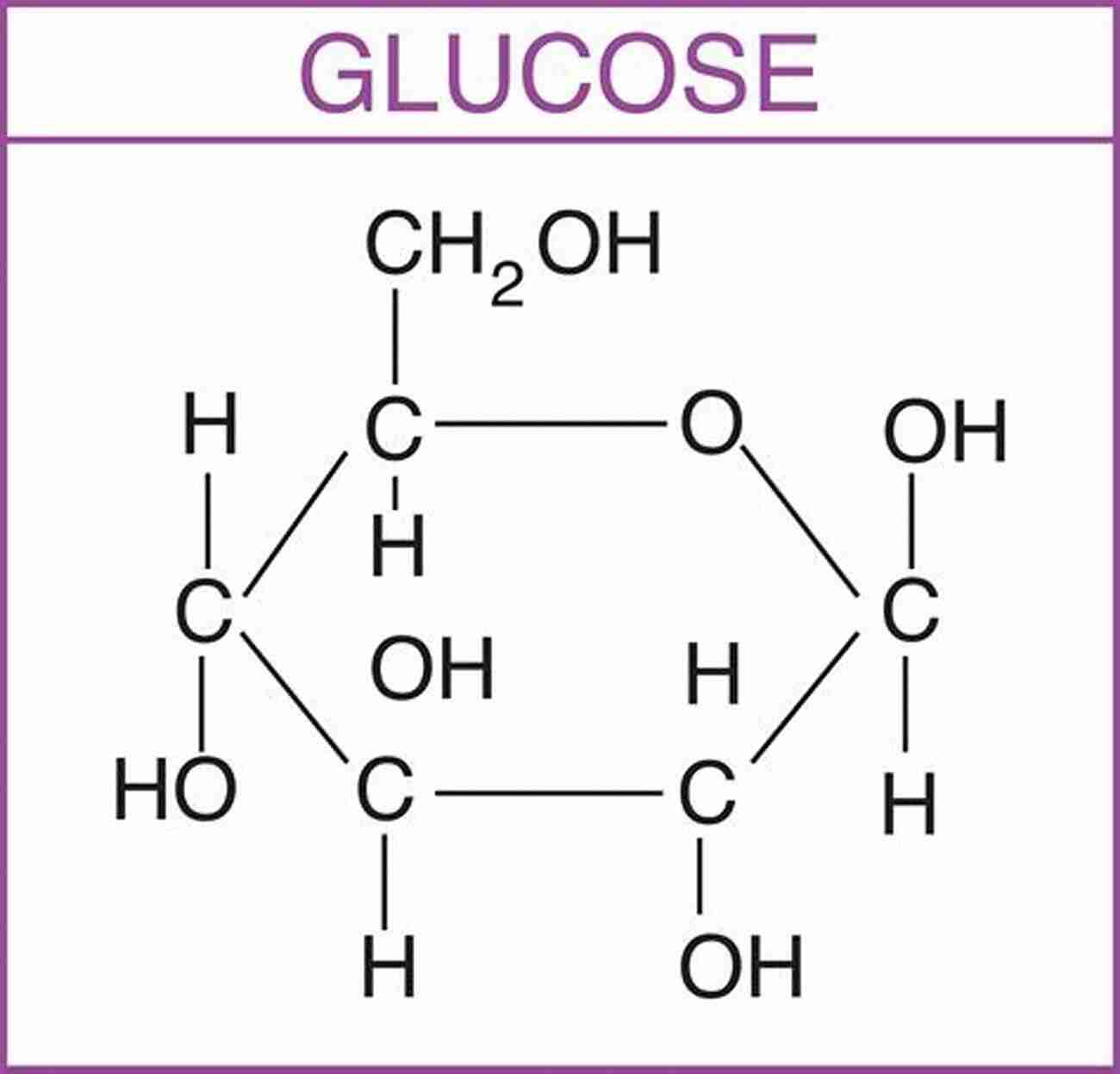
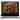
Image: Close-up of sugar molecules forming a carbohydrate
5 out of 5
| Language | : | English |
| File size | : | 3403 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 208 pages |
Carbohydrates
Carbohydrates, often referred to as sugars, are the main source of energy for living cells. They consist of carbon, hydrogen, and oxygen atoms and are classified as monosaccharides, disaccharides, or polysaccharides based on the number of sugar units they contain. These molecules undergo complex chemical reactions to release energy, allowing cells to carry out their functions.
Lipids
Lipids, including fats and oils, serve as energy reserves and insulation in the body. Moreover, they play a critical role in cell membranes, protecting cells and regulating their internal environment. Lipids are composed of carbon, hydrogen, and oxygen atoms, but their structure varies widely, leading to diverse functions within the body.
Proteins
Proteins are the workhorses of life, performing a vast array of functions within cells. They are involved in catalyzing chemical reactions, regulating gene expression, and transporting molecules within the body. The intricate structure of proteins, formed by amino acids, allows them to carry out their specific roles effectively.
Nucleic Acids
Nucleic acids, such as DNA and RNA, contain the genetic information necessary for the development and functioning of all known living organisms. These molecules are made up of nucleotides that store and transmit the instructions required for the synthesis of proteins and the reproduction of cells. The study of nucleic acids has revolutionized our understanding of genetics and heredity.
Physical Forces Driving Biochemical Processes
Behind the complex behaviors of biomolecules lies a multitude of physical forces that govern their interactions and determine their functions. Let's explore some of the fundamental principles that underpin biochemical processes.
Electromagnetic Interactions
The electromagnetic force, which encompasses both electrical and magnetic forces, plays a vital role in biochemical processes. It governs how charged atoms and molecules interact with one another, such as the attraction between positively charged protons and negatively charged electrons. Electromagnetic interactions regulate the formation and stability of chemical bonds, enabling the synthesis and breakdown of molecules within living systems.
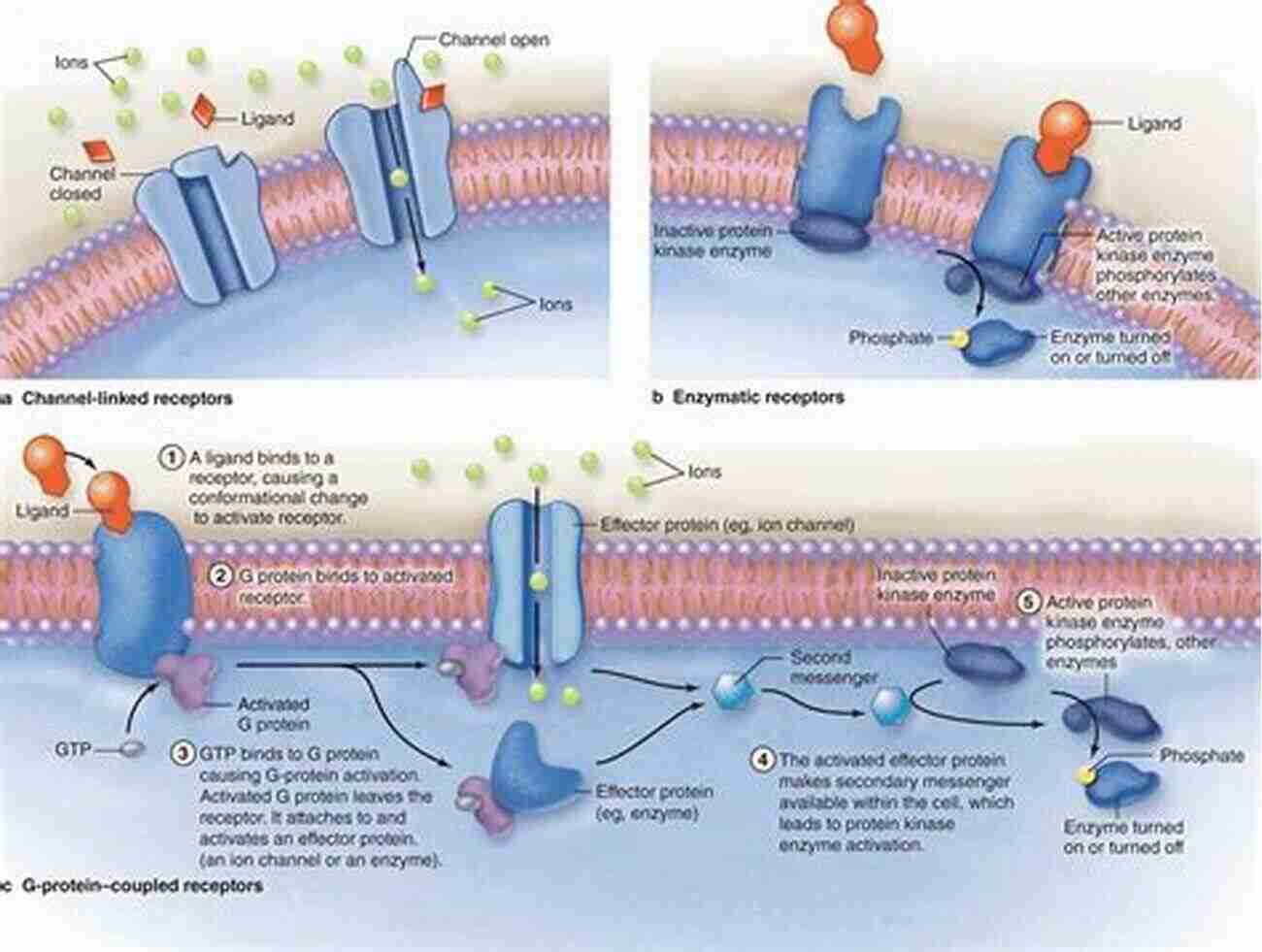
Hydrogen Bonding
Hydrogen bonds are key players in the formation and stabilization of complex biomolecular structures. These bonds occur when a hydrogen atom, bonded to an electronegative atom, forms a weak electrostatic interaction with another electronegative atom. Hydrogen bonding allows for the specific recognition and binding of molecules, crucial for various biological processes such as enzyme-substrate interactions and DNA replication.
Van der Waals Interactions
Van der Waals forces are weak intermolecular interactions that arise due to temporary fluctuations in electron density within atoms or molecules. These interactions are responsible for the close packing of biomolecules and the formation of 3D structures. They are particularly important in the folding of proteins and the binding of small ligands to proteins.
Experimental Techniques Shaping the Field
The study of biochemistry is heavily reliant on advanced experimental techniques that allow scientists to observe and analyze the intricate processes occurring within living systems. Let's explore some of the groundbreaking methods that have shaped the field.
X-ray Crystallography
X-ray crystallography is a powerful tool used to determine the atomic and molecular structure of a crystal. By exposing a crystal to X-rays and analyzing the diffraction patterns produced, scientists can unravel the precise arrangement of atoms within a molecule. This technique has been instrumental in uncovering the structures of proteins, nucleic acids, and other complex biomolecules.
Nuclear Magnetic Resonance (NMR)
NMR spectroscopy relies on the behavior of atomic nuclei in the presence of a strong magnetic field. By analyzing the magnetic properties of atomic nuclei, scientists can gain insights into the structure, dynamics, and interactions of biomolecules. NMR has been instrumental in characterizing protein structures and elucidating their functions.
Cryo-Electron Microscopy (Cryo-EM)
Cryo-EM is a cutting-edge technique that allows scientists to image biological molecules and complexes at high resolution. By freezing samples in a vitreous ice-like state and bombarding them with a beam of electrons, cryo-EM captures the 3D structure of biomolecules. This technique has revolutionized structural biology, enabling the visualization of intricate macromolecular assemblies.
The Future of Biochemistry
The physical basis of biochemistry continues to unravel the intricate machinery of life, offering invaluable insights into the functioning of living organisms. As technology advances and new experimental techniques emerge, our understanding of biochemistry will only deepen. The application of this knowledge holds tremendous potential for developing innovative medical therapies, biotechnological solutions, and sustainable approaches to address global challenges.
The physical basis of biochemistry lies at the intersection of chemistry and biology, providing a deeper understanding of the fundamental processes driving life itself. From the building blocks of life to the intricate forces governing biochemical reactions, this field opens doors to exploring the fascinating world of living organisms. As our understanding of the physical principles governing biochemistry expands, so does the potential for significant advancements, leading to a healthier and more sustainable future.
5 out of 5
| Language | : | English |
| File size | : | 3403 KB |
| Text-to-Speech | : | Enabled |
| Screen Reader | : | Supported |
| Enhanced typesetting | : | Enabled |
| Word Wise | : | Enabled |
| Print length | : | 208 pages |
advanced undergraduate/beginning graduate level students and would be applied to courses focusing on three different areas:
Foundations of molecular biophysics
Macromolecular structure and assembly
Methods in physical biochemistry

 Calvin Fisher
Calvin FisherThe Most Insightful and Liberating Experiences Found in...
When it comes to expanding our...

 D'Angelo Carter
D'Angelo CarterDax To The Max Imagination: Unlock the Power of...
Welcome to the world of Dax To...

 Chris Coleman
Chris ColemanThe Hidden Case of Ewan Forbes: Uncovering the Mystery...
Ewan Forbes: a...

 Morris Carter
Morris CarterWhen Newport Beat New Zealand: A Historic Rugby Upset
The rivalry between Newport and New Zealand...

 David Mitchell
David MitchellThe Soul of an Astronomer: Women of Spirit
Astronomy, the study of...

 Ethan Gray
Ethan GrayThe Military Origins Of The Republic 1763-1789
When we think about the birth of the...

 Guy Powell
Guy PowellRPO System for 10 and 11 Personnel: Durell Fain
When it comes to...

 Evan Hayes
Evan HayesMadness: The Ten Most Memorable NCAA Basketball Finals
College basketball fans eagerly await the...

 Jorge Amado
Jorge AmadoDiscover the Magic of Polish: English First 100 Words,...
Are you ready to embark on a linguistic...

 Shaun Nelson
Shaun NelsonUnlock the Secrets of Edwidge Danticat's Breath, Eyes,...
Are you delving into the world...

 Walt Whitman
Walt Whitman300 Years Liechtenstein: The Birth of Fish Out of Water...
Once upon a time, in the...

 Jaden Cox
Jaden CoxExploring the Legendary Surfers of Early Surfing in the...
Surfing, a sport...
Light bulbAdvertise smarter! Our strategic ad space ensures maximum exposure. Reserve your spot today!

 Roberto BolañoUnderstanding How Systemic Racism Created An American Social Construct Liable
Roberto BolañoUnderstanding How Systemic Racism Created An American Social Construct Liable
 Devon MitchellBeginners Guide To Growing Organic Vegetables Including Top Ten Easy Veg To...
Devon MitchellBeginners Guide To Growing Organic Vegetables Including Top Ten Easy Veg To...
 Roger TurnerDiscover the Tranquil Beauty of Whispering Pines in Your Summer State of Mind
Roger TurnerDiscover the Tranquil Beauty of Whispering Pines in Your Summer State of Mind
 Jonathan Franzen35am Five Nights At Freddy: Journey Through Sleepless Nights and Hair-Raising...
Jonathan Franzen35am Five Nights At Freddy: Journey Through Sleepless Nights and Hair-Raising... Miguel de CervantesFollow ·2.2k
Miguel de CervantesFollow ·2.2k Daniel KnightFollow ·10k
Daniel KnightFollow ·10k Efrain PowellFollow ·5.8k
Efrain PowellFollow ·5.8k Aleksandr PushkinFollow ·19.8k
Aleksandr PushkinFollow ·19.8k Keith CoxFollow ·16.7k
Keith CoxFollow ·16.7k Clayton HayesFollow ·13.8k
Clayton HayesFollow ·13.8k John GreenFollow ·14.2k
John GreenFollow ·14.2k Stephen KingFollow ·14k
Stephen KingFollow ·14k














